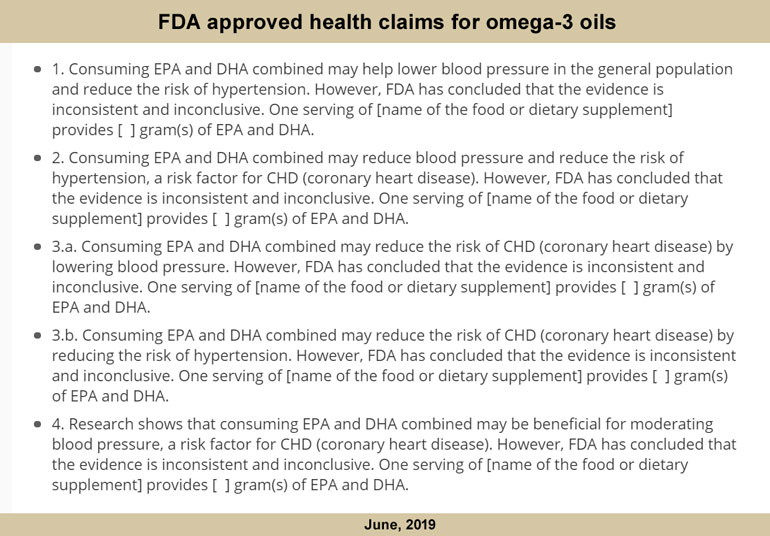
1. Supportive, but not conclusive research (lowest level)
2. Credible evidence
3. Significant scientific agreement.
“I think the approval of the health claims is a welcome step forward in the recognition of the multiple health benefits of omega-3 fatty acids. It’s not just about their effects on blood lipids, like triglycerides,” said Harris. “The do affect other risk factors, like blood pressure and endothelial function.”
If you’re convinced of the benefits of essential fatty acids, including omega-3s, check out Optimal E.F.A from Optimal Health Systems.
Optimal E.F.A provides potent essential fatty acids via all-vegetarian sources—and provides them in the correct ratio of omega-3s and omega-6s.
– – –
Sources: Regulations.gov, NutraIngredients.com, GOEDomega3.com.